Level 01

Level 02
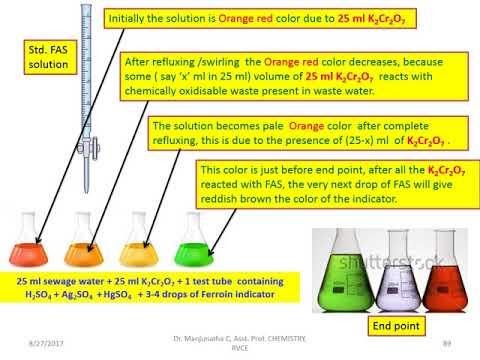
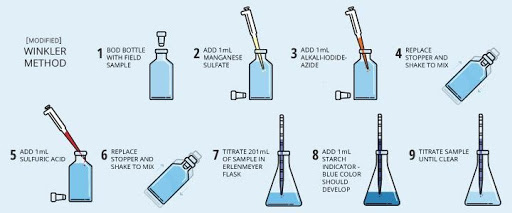
Water pollution can be determined by the amount of dissolved oxygen. The amount of dissolved oxygen in a water sample tells us about the quality of water. There are two dissolved oxygen scales, Biological Oxygen Demand (BOD) and Chemical Oxygen Demand (COD).

Level 03
Level 04
Level 05
Select a strong oxidising agent to be put in burette.
?Select the oxidising agents from given list.





Level 06
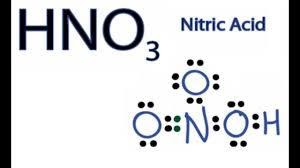
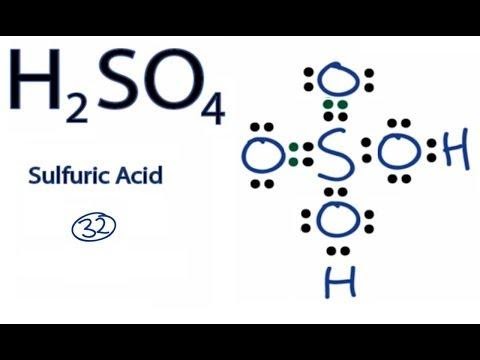
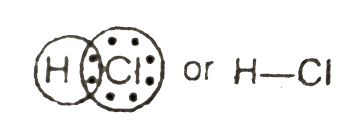
To change from Cr (+6) to Cr (+3) oxidation state, dichromate needs a lot of acid. What do you think, which is the most suitable acid?
?Select the acid
Level 07
In COD determination, the chloride ions present in water cause interference in the estimation
?Fill in the blanks
3Cl2
3Cr
3H2
?So, to get rid of that which reagent must be added?





Level 08
The oxidizing agent dichromate oxidizes organic and inorganic matter in the sample. After digestion, the amount of excess potassium dichromate will give the amount of potassium dichromate got used up in the oxidation of waste matter in water.
?Select the burette solution which will react with the excess dichromate solution.






